
-
 Swiss 'Mountain Tinder' sparks high-altitude attraction
Swiss 'Mountain Tinder' sparks high-altitude attraction
-
Hong Kong hit by flooding after flurry of rainstorm warnings

-
 Asian markets track Wall St rally on Fed rate cut bets
Asian markets track Wall St rally on Fed rate cut bets
-
Gaza war deepens Israel's divides

-
 Beijing lifts rain alert after evacuating over 80,000
Beijing lifts rain alert after evacuating over 80,000
-
Decision time as plastic pollution treaty talks begin

-
 Zverev ignores fan distraction to advance to ATP Toronto semis
Zverev ignores fan distraction to advance to ATP Toronto semis
-
Remains of 32 people found in Mexico's Guanajuato state

-
 Trump tariffs don't spare his fans in EU
Trump tariffs don't spare his fans in EU
-
Brazil judge puts ex-president Bolsonaro under house arrest

-
 With six months to go, Winter Games organisers say they'll be ready
With six months to go, Winter Games organisers say they'll be ready
-
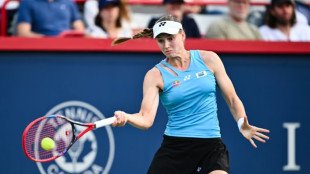
Rybakina to face teen Mboko in WTA Canadian Open semis
-
 Australia to buy 11 advanced warships from Japan
Australia to buy 11 advanced warships from Japan
-
Five years after Beirut port blast, Lebanese demand justice

-
 Stella Rimington, first woman to lead UK's MI5 dies at 90
Stella Rimington, first woman to lead UK's MI5 dies at 90
-
Trump admin to reinstall Confederate statue toppled by protesters
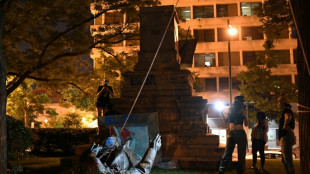
-
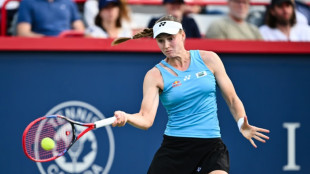 Rybakina advances to WTA Canadian Open semis
Rybakina advances to WTA Canadian Open semis
-
Brazilian judge places ex-president Bolsonaro under house arrest

-
 Brazil judge places ex-president Bolsonaro under house arrest
Brazil judge places ex-president Bolsonaro under house arrest
-
NGOs caught between juntas and jihadists in turbulent Sahel

-
 NBA Spurs agree to four-year extension with Fox: reports
NBA Spurs agree to four-year extension with Fox: reports
-
Stocks mostly rebound on US interest rate cut bets

-
 Boeing defense workers launch strike over contract dispute
Boeing defense workers launch strike over contract dispute
-
Grand Canyon fire rages, one month on

-
 Djokovic withdraws from ATP Cincinnati Masters
Djokovic withdraws from ATP Cincinnati Masters
-
Brazil's Paixao promises 'big things' at Marseille unveiling

-
 Shubman Gill: India's elegant captain
Shubman Gill: India's elegant captain
-
Trump says to name new labor statistics chief this week

-
 England v India: Three talking points
England v India: Three talking points
-
Exceptional Nordic heatwave stumps tourists seeking shade

-
 'Musical cocoon': Polish mountain town hosts Chopin fest
'Musical cocoon': Polish mountain town hosts Chopin fest
-
A 'Thinker' drowns in plastic garbage as UN treaty talks open

-
 India's Siraj 'woke up believing' ahead of Test heroics
India's Siraj 'woke up believing' ahead of Test heroics
-
Israeli PM says to brief army on Gaza war plan

-
 Frustrated Stokes refuses to blame Brook for England collapse
Frustrated Stokes refuses to blame Brook for England collapse
-
Moscow awaits 'important' Trump envoy visit before sanctions deadline

-
 Schick extends Bayer Leverkusen contract until 2030
Schick extends Bayer Leverkusen contract until 2030
-
Tesla approves $29 bn in shares to Musk as court case rumbles on

-
 Stocks rebound on US rate cut bets
Stocks rebound on US rate cut bets
-
Swiss eye 'more attractive' offer for Trump after tariff shock

-
 Trump says will name new economics data official this week
Trump says will name new economics data official this week
-
Three things we learned from the Hungarian Grand Prix

-
 Lions hooker Sheehan banned over Lynagh incident
Lions hooker Sheehan banned over Lynagh incident
-
Jordan sees tourism slump over Gaza war

-
 China's Baidu to deploy robotaxis on rideshare app Lyft
China's Baidu to deploy robotaxis on rideshare app Lyft
-
Israel wants world attention on hostages held in Gaza

-
 Pacific algae invade Algeria beaches, pushing humans and fish away
Pacific algae invade Algeria beaches, pushing humans and fish away
-
Siraj stars as India beat England by six runs in fifth-Test thriller

-
 Stocks mostly rise as traders boost US rate cut bets
Stocks mostly rise as traders boost US rate cut bets
-
S.Africa eyes new markets after US tariffs: president

| SCU | 0% | 12.72 | $ | |
| RBGPF | 0% | 74.94 | $ | |
| BCC | -0.77% | 82.71 | $ | |
| NGG | 1.14% | 72.65 | $ | |
| CMSD | 1.18% | 23.63 | $ | |
| AZN | 0.86% | 74.59 | $ | |
| BCE | -1.12% | 23.31 | $ | |
| RIO | 0.58% | 60 | $ | |
| RELX | 0.73% | 51.97 | $ | |
| CMSC | 0.87% | 23.07 | $ | |
| GSK | 0.32% | 37.68 | $ | |
| RYCEF | 2.14% | 14.5 | $ | |
| JRI | 0.76% | 13.2 | $ | |
| SCS | 38.6% | 16.58 | $ | |
| BTI | 2.16% | 55.55 | $ | |
| VOD | 0.72% | 11.04 | $ | |
| BP | 2.28% | 32.49 | $ |

BioNxt Solutions Reports Formal Notice from the European Patent Office of Intention to Grant Patent
VANCOUVER, BC / ACCESS Newswire / May 23, 2025 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTC PINK:BNXTF)(FSE:BXT), a bioscience innovator specializing in advanced drug delivery systems, is pleased to announce that the examining division of the European Patent Office ("EPO") has provided formal notice of the EPO's intention to grant BioNxt its core patent without significant changes.
The Company's core patent filing was a comprehensive application for the sublingual delivery of anticancer drugs for the treatment of autoimmune neurodegenerative diseases. This patent family provides numerous proprietary product development and commercialization opportunities, including BioNxt's lead product, BNT23001, a sublingual thin-film formulation of Cladribine for the treatment of multiple sclerosis (MS).
"Confirmation of the Company's flagship intellectual property asset in Europe is a major milestone for BioNxt," stated Hugh Rogers, CEO of BioNxt. "We expect the European patent grant to be finalized and published in the coming weeks. The timing is excellent as we can confidently prepare for the upcoming BNT23001 human bioequivalence study in Europe."
BioNxt continues to advance the nationalization phase of the patent protection process at the European Patent Office and Eurasian Patent Organization, as well as with independent filing nations, such as Australia (AU), Canada (CA), New Zealand (NZ), USA (US), and Japan (JP). Securing nation-level patents around the globe will serve as the foundation for commercial opportunities for the Company's pipeline of sublingual products targeting autoimmune diseases such as multiple sclerosis (MS), myasthenia gravis (MG), lupus nephritis (LN) and rheumatoid arthritis (RA).
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next-generation drug delivery technologies, diagnostic screening systems, and active pharmaceutical ingredient development. The Company's proprietary platforms-Sublingual (Thin-Film), Transdermal (Skin Patch), and Oral (Enteric-Coated Tablets)-target key therapeutic areas, including autoimmune diseases, neurological disorders, and longevity.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient-centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co-Founder, CEO and Director
Email: [email protected]
Phone: +1 778.598.2698
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt-solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward-Looking" Information
This press release contains forward-looking statements within the meaning of applicable securities laws, including statements regarding the development, testing, regulatory approval, and commercialization of BioNxt's sublingual drug products, as well as projected milestones, anticipated partnerships, and potential market opportunities. Forward-looking statements are inherently subject to significant risks, uncertainties, and assumptions, many of which are beyond BioNxt's control. Factors that could cause actual results to differ materially include, but are not limited to, delays in regulatory approvals, negative outcomes from clinical trials, changes in market demand, fluctuations in funding availability, or disruptions in supply chains. Readers are cautioned not to place undue reliance on these forward-looking statements, as actual results may differ materially from those expressed or implied. BioNxt undertakes no obligation to update or revise forward-looking statements, except as required by law. Factors that could cause actual results to differ materially from those projected include changes in market demand, regulatory developments, delays in clinical trials, fluctuations in financing availability, supply chain disruptions, and unforeseen competitive pressures.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
B.AbuZeid--SF-PST
