-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse

-
 Timber hopes League Cup can be catalyst for Arsenal success
Timber hopes League Cup can be catalyst for Arsenal success
-
China calls EU 'discriminatory' over probe into energy giant Goldwind

-
 Sales warning slams Ozempic maker Novo Nordisk's stock
Sales warning slams Ozempic maker Novo Nordisk's stock
-
Can Vonn defy ACL rupture to win Olympic medal?

-
 Breakthrough or prelude to attack? What we know about Iran-US talks
Breakthrough or prelude to attack? What we know about Iran-US talks
-
German far-right MP detained over alleged Belarus sanctions breach

-
 MSF says its hospital in South Sudan hit by government air strike
MSF says its hospital in South Sudan hit by government air strike
-
Merz heads to Gulf as Germany looks to diversify trade ties

-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

-
 Gaza health officials say strikes kill 21 after Israel says shots wounded officer
Gaza health officials say strikes kill 21 after Israel says shots wounded officer
-
Injured Vonn's Olympic bid is 'inspirational', ski stars say

-
 Albania arrests 20 for toxic waste trafficking
Albania arrests 20 for toxic waste trafficking
-
US-Africa trade deal renewal only 'temporary breather'

-
 Mir sets pace on Sepang day two, Yamaha absent
Mir sets pace on Sepang day two, Yamaha absent
-
Xi, Putin hail 'stabilising' China-Russia alliance

-
 GSK boosted by specialty drugs, end to Zantac fallout
GSK boosted by specialty drugs, end to Zantac fallout
-
UK's ex-prince leaves Windsor home amid Epstein storm: reports

| RYCEF | -2.1% | 16.65 | $ | |
| RBGPF | 0.12% | 82.5 | $ | |
| CMSC | -0.6% | 23.52 | $ | |
| CMSD | -0.38% | 23.85 | $ | |
| VOD | 2.44% | 15.631 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| BCC | 4.95% | 89.35 | $ | |
| RIO | -1.38% | 95.06 | $ | |
| NGG | 2.14% | 88.12 | $ | |
| JRI | -0.17% | 13.098 | $ | |
| RELX | -2.26% | 29.835 | $ | |
| BCE | 0.97% | 26.355 | $ | |
| GSK | 7.07% | 57.4 | $ | |
| AZN | 2.81% | 189.64 | $ | |
| BTI | -0.32% | 61.67 | $ | |
| BP | 0.63% | 39.065 | $ |

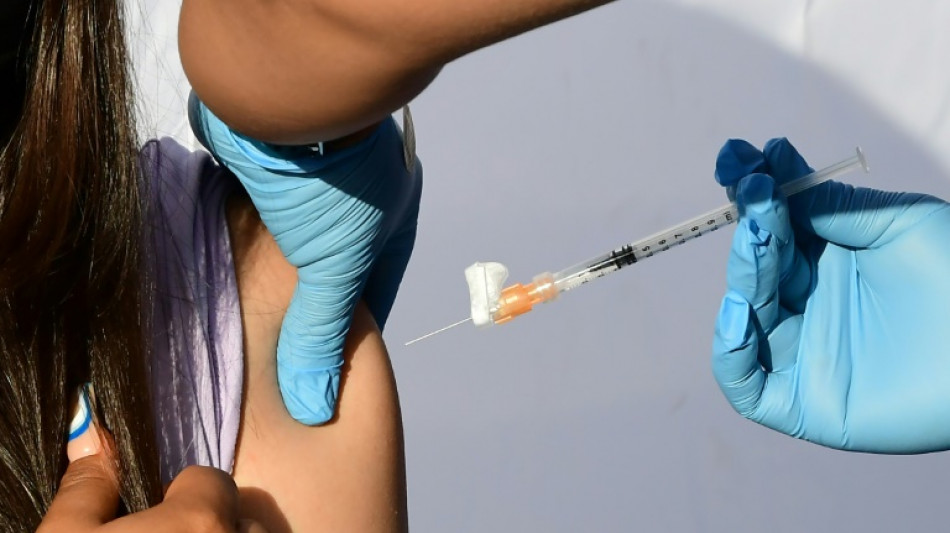
US panel weighs authorizing Covid vaccines for youngest children
After months of anxious wait for some parents, a panel of experts convened by the US Food and Drug Administration will meet Wednesday to weigh recommending Covid vaccines for the nation's youngest children.
Children under five are the only age group not yet eligible for Covid immunization in the United States and most other countries. If, as expected, panelists vote in favor of greenlighting the Pfizer and Moderna vaccines, formal authorizations should follow quickly, with the first shots in arms expected by next week.
Ahead of the meeting, the FDA posted its independent analyses of the two drugs, deeming both safe and effective. It also said there was a pressing unmet need to inoculate children in the age group since their rates of hospitalization and death "are higher than among children and adolescents 5-17 years of age."
Both vaccines are based on messenger RNA technology, which delivers genetic code for the coronavirus spike protein to human cells, training the immune system to be ready for when it encounters the real virus.
Pfizer is seeking authorization for three doses at three micrograms given to ages six months through four years, while Moderna has asked for the FDA to approve its vaccine as two doses of a higher 30 micrograms, for ages six months through five years.
They were tested in trials of thousands of children where they were found to have similar levels of mild effects to older age groups and triggered similar levels of antibodies.
Efficacy against infection was higher for Pfizer, with the company placing it at 80 percent compared to Moderna's estimates of 51 percent in the six-month-old to age two group and 37 percent in the two to five years age group.
But the figures are provisional and Moderna is studying adding a third dose later that may increase its figures.
There are some 20 million US children aged four years and under. If the FDA-appointed experts recommend the two vaccines, then the matter will go to another committee convened by the Centers for Disease Control and Prevention for a final say.
White House officials last week said the rollout of millions of shots at pharmacies and doctors' offices could begin as soon as June 21, following the Juneteenth holiday on June 20.
Of the total US Covid deaths, 480 have come in children under five, according to the latest official data.
Obesity, neurological disorders and asthma are associated with increased risk of severe disease, "however, a majority of children hospitalized for Covid-19 have no underlying medical conditions," the FDA said in a document.
Children can also go on to contract multisystem inflammatory syndrome in children (MIS-C), a rare but serious post-viral condition.
Data on long Covid in children is sparse, but the FDA's document cited a national survey in the United Kingdom which found that "among children ages two to 11 years who tested positive for COVID-19, 7.2 percent reported continued symptoms at 12 weeks."
J.AbuHassan--SF-PST



