-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
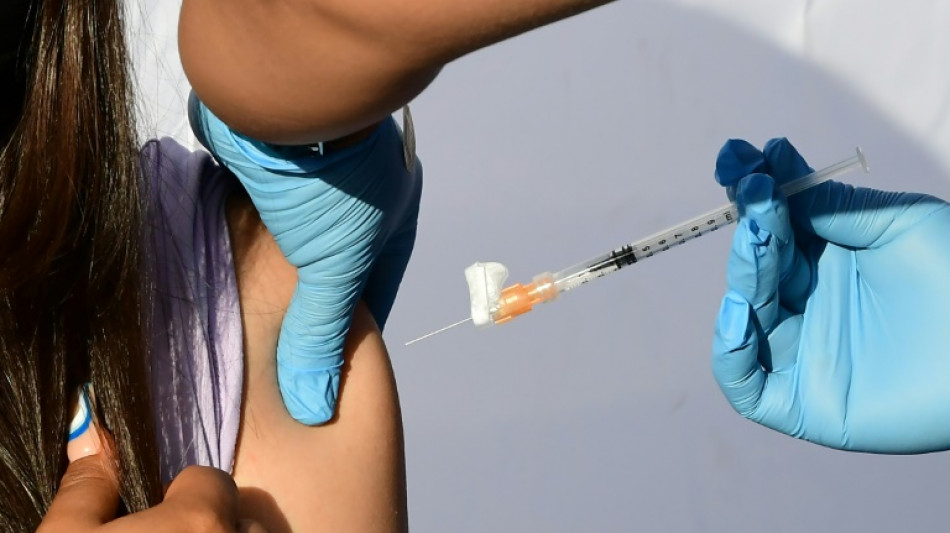
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse

-
 Timber hopes League Cup can be catalyst for Arsenal success
Timber hopes League Cup can be catalyst for Arsenal success
-
China calls EU 'discriminatory' over probe into energy giant Goldwind

-
 Sales warning slams Ozempic maker Novo Nordisk's stock
Sales warning slams Ozempic maker Novo Nordisk's stock
-
Can Vonn defy ACL rupture to win Olympic medal?

-
 Breakthrough or prelude to attack? What we know about Iran-US talks
Breakthrough or prelude to attack? What we know about Iran-US talks
-
German far-right MP detained over alleged Belarus sanctions breach

-
 MSF says its hospital in South Sudan hit by government air strike
MSF says its hospital in South Sudan hit by government air strike
-
Merz heads to Gulf as Germany looks to diversify trade ties

-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

-
 Gaza health officials say strikes kill 21 after Israel says shots wounded officer
Gaza health officials say strikes kill 21 after Israel says shots wounded officer
-
Injured Vonn's Olympic bid is 'inspirational', ski stars say

-
 Albania arrests 20 for toxic waste trafficking
Albania arrests 20 for toxic waste trafficking
-
US-Africa trade deal renewal only 'temporary breather'

-
 Mir sets pace on Sepang day two, Yamaha absent
Mir sets pace on Sepang day two, Yamaha absent
-
Xi, Putin hail 'stabilising' China-Russia alliance

-
 GSK boosted by specialty drugs, end to Zantac fallout
GSK boosted by specialty drugs, end to Zantac fallout
-
UK's ex-prince leaves Windsor home amid Epstein storm: reports

| RYCEF | -2.1% | 16.65 | $ | |
| RBGPF | 0.12% | 82.5 | $ | |
| CMSC | -0.6% | 23.52 | $ | |
| CMSD | -0.38% | 23.85 | $ | |
| VOD | 2.44% | 15.631 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| BCC | 4.95% | 89.35 | $ | |
| RIO | -1.38% | 95.06 | $ | |
| NGG | 2.14% | 88.12 | $ | |
| JRI | -0.17% | 13.098 | $ | |
| RELX | -2.26% | 29.835 | $ | |
| BCE | 0.97% | 26.355 | $ | |
| GSK | 7.07% | 57.4 | $ | |
| AZN | 2.81% | 189.64 | $ | |
| BTI | -0.32% | 61.67 | $ | |
| BP | 0.63% | 39.065 | $ |

US FDA says Pfizer Covid vaccine effective in kids under five
The US Food and Drug Administration (FDA) has said the Pfizer Covid vaccine is safe and effective in children under five, ahead of a meeting to weigh its authorization later this week.
Children under five are the only age group not yet eligible for vaccination in the United States and most countries, a pressing need since rates of hospitalization and death "are higher than among children and adolescents 5-17 years of age," the FDA said in a document posted on its website Sunday.
The agency has called a meeting of experts on June 15 to decide whether to recommend the Pfizer vaccine, given as three shots to children aged six months through four years, as well as the Moderna vaccine, given as two shots to children aged six months through five years.
Pfizer's first two shots are given three weeks apart, then the third is given eight weeks after the second. They are all dosed at three micrograms, as opposed to 30 micrograms the company gives older ages.
Both Pfizer and Moderna had previously posted their results, but the FDA then had to review the data in detail and carry out its own evaluation. It posted a favorable analysis about Moderna on Friday.
Its comments towards Pfizer also appear favorable, based on the levels of infection-blocking antibodies it evoked in trial participants, and a similar side-effect profile to higher age groups. The trial population was around 4,500 children.
A preliminary estimate placed vaccine efficacy at 80.3 percent, but the FDA noted this was based on very few positive cases -- just 10, as opposed to the 21 sought for a more accurate figure.
There are nearly 20 million US children aged four years and under, or six percent of the population. If, as expected, the FDA-appointed experts recommend the two shots, then the matter will go to another committee convened by the Centers for Disease Control and Prevention for a final say.
White House officials last week said rollout of millions of shots at pharmacies and doctors' offices could begin as soon as June 21, following the Juneteenth holiday on June 20.
E.AbuRizq--SF-PST



