-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse

-
 Timber hopes League Cup can be catalyst for Arsenal success
Timber hopes League Cup can be catalyst for Arsenal success
-
China calls EU 'discriminatory' over probe into energy giant Goldwind

-
 Sales warning slams Ozempic maker Novo Nordisk's stock
Sales warning slams Ozempic maker Novo Nordisk's stock
-
Can Vonn defy ACL rupture to win Olympic medal?

-
 Breakthrough or prelude to attack? What we know about Iran-US talks
Breakthrough or prelude to attack? What we know about Iran-US talks
-
German far-right MP detained over alleged Belarus sanctions breach

-
 MSF says its hospital in South Sudan hit by government air strike
MSF says its hospital in South Sudan hit by government air strike
-
Merz heads to Gulf as Germany looks to diversify trade ties

-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

-
 Gaza health officials say strikes kill 21 after Israel says shots wounded officer
Gaza health officials say strikes kill 21 after Israel says shots wounded officer
-
Injured Vonn's Olympic bid is 'inspirational', ski stars say

-
 Albania arrests 20 for toxic waste trafficking
Albania arrests 20 for toxic waste trafficking
-
US-Africa trade deal renewal only 'temporary breather'

-
 Mir sets pace on Sepang day two, Yamaha absent
Mir sets pace on Sepang day two, Yamaha absent
-
Xi, Putin hail 'stabilising' China-Russia alliance

-
 GSK boosted by specialty drugs, end to Zantac fallout
GSK boosted by specialty drugs, end to Zantac fallout
-
UK's ex-prince leaves Windsor home amid Epstein storm: reports

-
 Sky is the limit for Ireland fly-half Prendergast, says captain Doris
Sky is the limit for Ireland fly-half Prendergast, says captain Doris
-
Stocks fluctuate after Wall St AI-fuelled sell-off

-
 Feyi-Waboso reminds England great Robinson of himself
Feyi-Waboso reminds England great Robinson of himself
-
Starmer faces MPs as pressure grows over Mandelson scandal

-
 HRW urges pushback against 'aggressive superpowers'
HRW urges pushback against 'aggressive superpowers'
-
Russia demands Ukraine give in as UAE talks open

-
 Gaza civil defence says 17 killed in strikes after Israel says shots wounded officer
Gaza civil defence says 17 killed in strikes after Israel says shots wounded officer
-
France's Kante joins Fenerbahce after Erdogan 'support'

| CMSD | -0.36% | 23.855 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| RBGPF | 0.12% | 82.5 | $ | |
| RYCEF | -1.19% | 16.8 | $ | |
| VOD | 2.34% | 15.615 | $ | |
| RIO | -0.86% | 95.55 | $ | |
| CMSC | -0.55% | 23.53 | $ | |
| NGG | 1.89% | 87.89 | $ | |
| BTI | -0.34% | 61.66 | $ | |
| BCE | 1.17% | 26.41 | $ | |
| BCC | 4.73% | 89.15 | $ | |
| RELX | -1.56% | 30.04 | $ | |
| GSK | 6.35% | 56.955 | $ | |
| JRI | 0.22% | 13.149 | $ | |
| BP | 0.77% | 39.12 | $ | |
| AZN | 1.18% | 186.53 | $ |

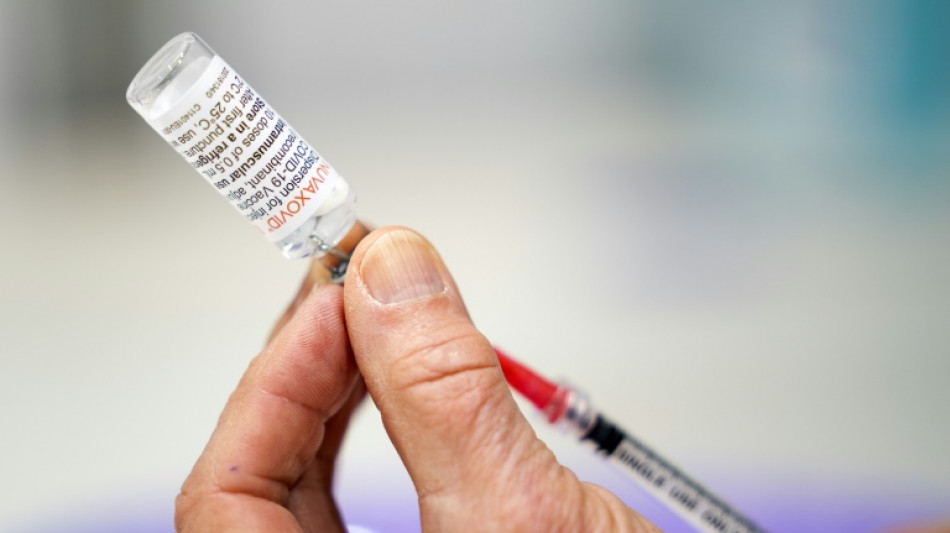
FDA experts weigh authorizing Novavax Covid-19 vaccine in US
A panel of experts convened by the US drug regulator was meeting Tuesday to consider authorizing the Novavax Covid-19 shot, a late runner in the fight against the virus that could nonetheless play a role in overcoming vaccine hesitancy.
Three vaccines are currently approved in the United States: Pfizer and Moderna, which are based on messenger RNA, and Johnson and Johnson, an adenovirus vector vaccine.
But the last of these, the J&J vaccine, was recently restricted in the US after being linked to a rare but serious clotting condition, especially in women of reproductive age.
It is now only recommended for adults who cannot access Pfizer or Moderna for medical or other serious reasons.
The Novavax vaccine was an early frontrunner in the vaccine race, but fell behind after being hit by manufacturing and regulatory delays.
Though the company is American, the US is one of the few major markets where it hasn't yet received authorization -- the EU, UK, Canada, Australia are among many that have already given it the green light.
Officials hope that the shot, which is based on synthetic proteins, could provide an alternative to the mRNA vaccines for people still hesitant.
"We do have a problem with vaccine uptake that is very serious in the United States," Peter Marks, a senior scientist for the Food and Drug administration, said at the start of the meeting.
"And anything we can do to get people more comfortable to be able to accept these potentially life saving medical products, is something that we feel we are compelled to do."
Of the various vaccine technologies, mRNA has been subject to the most misinformation efforts.
Novavax's vaccine was found to be 90 percent effective against symptomatic cases of the disease, in trials conducted before the appearance of the Omicron variant, according to the FDA.
But six cases of myocarditis, an inflammation of the heart muscle, were detected in the group that received the vaccine, against one case in the placebo group, in a trial of around 40,000 people.
Novavax says there is insufficient evidence to establish a causal relationship between the cases of myocarditis and the vaccine.
The FDA voiced concern over the myocarditis link on Friday, sending Novavax shares to drop 20 percent on the New York Stock Exchange. And trading of Novavax stock was halted on Monday pending news from the FDA panel.
Known as a protein subunit vaccine, Novavax is administered in two doses.
It uses a synthetic version of the virus' spike protein to evoke an immune response.
The same technique is used in vaccines against whooping cough, meningococcal meningitis and hepatitis B.
K.AbuTaha--SF-PST




