-
 Scandic Trust Group strengthens sales network with First Idea Consultant
Scandic Trust Group strengthens sales network with First Idea Consultant
-
Kolisi 100th Test 'no distraction' for Erasmus' South Africa

-
 Teetering Belgian government given more time to agree budget
Teetering Belgian government given more time to agree budget
-
Merz backs EU plan to protect steel sector from Chinese imports

-
 New Zealand make Scotland changes after Barrett brothers' injuries
New Zealand make Scotland changes after Barrett brothers' injuries
-
'Roy of the Rovers story' -- Farrell handed Ireland debut for Japan Test

-
 Stones backs Man City team-mate Foden to pose England dilemma for Tuchel
Stones backs Man City team-mate Foden to pose England dilemma for Tuchel
-
Djokovic to face Alcaraz in ATP Finals groups

-
 Facing climate 'overshoot', world heads into risky territory
Facing climate 'overshoot', world heads into risky territory
-
Springbok skipper Kolisi to play 100th Test against France

-
 Typhoon Kalmaegi hits Vietnam after killing 140 in Philippines
Typhoon Kalmaegi hits Vietnam after killing 140 in Philippines
-
Bank of England leaves rate unchanged before UK budget

-
 Germany recall Sane, hand El Mala debut for World Cup qualifers
Germany recall Sane, hand El Mala debut for World Cup qualifers
-
India thump Australia to take 2-1 lead in T20 series

-
 Cameroon's Biya, world's oldest president, sworn in for 8th term
Cameroon's Biya, world's oldest president, sworn in for 8th term
-
Flick holding firm on Barca high line despite defensive woes

-
 Battered US businesses eye improved China trade at Shanghai expo
Battered US businesses eye improved China trade at Shanghai expo
-
France opt for Le Garrec as Dupont replacement for 'best team ever' South Africa

-
 Drugmaker AstraZeneca profit jumps as US business grows
Drugmaker AstraZeneca profit jumps as US business grows
-
'Vibe coding' named word of the year by Collins dictionary

-
 Vietnam evacuates thousands from coast ahead of Typhoon Kalmaegi
Vietnam evacuates thousands from coast ahead of Typhoon Kalmaegi
-
European stocks fall after gains in Asia, US

-
 MotoGP legend Agostini admires Marc Marquez's 'desire to win'
MotoGP legend Agostini admires Marc Marquez's 'desire to win'
-
Nepal searches for avalanche victims

-
 Hezbollah rejects any negotiations between Lebanon and Israel
Hezbollah rejects any negotiations between Lebanon and Israel
-
Chapman blitz leads Black Caps to tight T20 victory over West Indies

-
 France urges EU to sanction Shein platform
France urges EU to sanction Shein platform
-
France opt for Le Garrec as Dupont replacement for South Africa Test

-
 Turmoil in tiaras at Miss Universe pageant in Thailand
Turmoil in tiaras at Miss Universe pageant in Thailand
-
Probe into Thales defence group looking at Indonesian contract

-
 US to cancel flights as longest govt shutdown drags on
US to cancel flights as longest govt shutdown drags on
-
Home in Nigeria, ex-refugees find themselves in a war zone

-
 Doncic's Lakers hold off Wembanyama's Spurs, Blazers silence Thunder
Doncic's Lakers hold off Wembanyama's Spurs, Blazers silence Thunder
-
For Turkey's LGBTQ community, draft law sparks existential alarm

-
 Musk's $1 trillion pay package to face Tesla shareholder vote
Musk's $1 trillion pay package to face Tesla shareholder vote
-
Tonga rugby league star out of intensive care after seizure

-
 Argentine ex-president Kirchner goes on trial in new corruption case
Argentine ex-president Kirchner goes on trial in new corruption case
-
Dams, housing, pensions: Franco disinformation flourishes online

-
 Endo returns as Japan look to build on Brazil win
Endo returns as Japan look to build on Brazil win
-
Franco captivates young Spaniards 50 years after death

-
 German steel industry girds for uncertain future
German steel industry girds for uncertain future
-
IPL champions Bengaluru could be sold for 'as much as $2 billion'

-
 Budget impasse threatens Belgium's ruling coalition
Budget impasse threatens Belgium's ruling coalition
-
New Zealand ex-top cop admits to having material showing child abuse, bestiality

-
 BoE set for finely balanced pre-budget rate call
BoE set for finely balanced pre-budget rate call
-
Australian kingpin obtains shorter sentence over drug charge

-
 Weatherald's unenviable Ashes task: fill giant hole at top left by Warner
Weatherald's unenviable Ashes task: fill giant hole at top left by Warner
-
Ovechkin first to score 900 NHL goals as Capitals beat Blues

-
 On Mexico City's streets, vendors fight to make it to World Cup
On Mexico City's streets, vendors fight to make it to World Cup
-
Asian markets bounce from selloff as US jobs beat forecasts


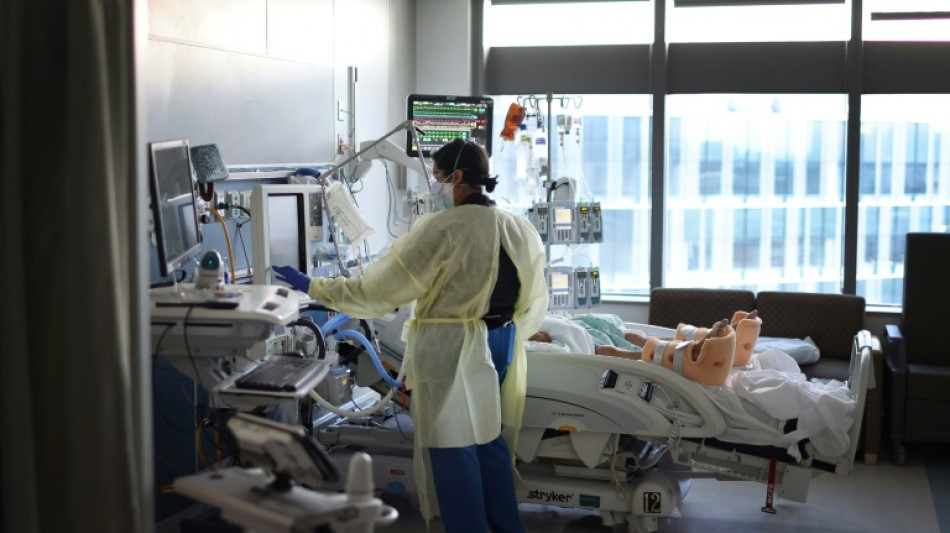
Trial of new Covid treatment yields encouraging results: study
A single-injection antiviral treatment for newly-infected Covid-19 patients reduced the risk of hospitalization by half in a large-scale clinical trial, according to a study published on Wednesday.
Stanford University professor Jeffrey Glenn, coauthor of the study published in the New England Journal of Medicine (NEJM), said the new drug "showed profound benefits for vaccinated and unvaccinated people alike."
While the number of Americans dying daily of coronavirus has fallen to around 500, treatments for the disease remain limited. One of the most common, Paxlovid made by Pfizer, involves taking 30 pills over five days.
The new treatment involves a single dose of pegylated lambda-interferon, a synthetic version of a naturally occurring protein that infected cells secrete to defend against viral infection.
"What it does is it binds receptors on the surfaces of cells that activate our own antiviral defense mechanisms," said Glenn, a professor of medicine, microbiology and immunology who heads the Stanford Biosecurity and Pandemic Preparedness Initiative.
"So if a virus has infected the cell it will turn on processes that aim to destroy the virus's replication," he said. "It will also send signals to neighboring cells to warn them viruses are on their way and get ready to defend yourself."
Receptors for interferon lambda are primarily in the linings of the lungs, airways and intestine -- the main places Covid strikes.
"We're turning on these antiviral mechanisms in the cells, the lung, where the infection is happening," Glenn said.
The Phase 3 trial of the drug, conducted between June 2021 and February 2022, involved nearly 2,000 patients with Covid symptoms in Brazil and Canada, about 85 percent of whom had been vaccinated.
A total of 931 newly-infected Covid patients were given a single injection of interferon lambda while 1,018 participants were given a placebo.
The risk of Covid-19–related hospitalization or death from any cause was 47 percent lower in the interferon group than in the placebo group, according to the researchers.
Twenty-five of the 931 people who received the injection within seven days of exhibiting Covid symptoms were hospitalized compared with 57 of the 1,018 who received the placebo.
Vaccinated patients treated with interferon lambda experienced a 51 percent reduction in hospitalization relative to the placebo group.
There was an 89 percent reduction in hospitalization among unvaccinated patients treated within the first three days of the onset of Covid symptoms compared with the placebo group.
- Developed for hepatitis D -
Glenn said interferon lambda proved effective against all Covid variants tested, including Omicron, and side effects in the group receiving the injections were no greater than among the placebo recipients.
Glenn is the founder of a small biotechnology company called Eiger Biopharmaceuticals that acquired interferon lambda to develop drugs for hepatitis delta virus.
"When Covid came, I said this would be the perfect drug for Covid," said Glenn, who left the Palo Alto-based company but remains on the board of directors and is an equity holder.
Eiger sought an emergency use authorization for interferon lambda from the US Federal Drug Administration (FDA) for Covid treatment last year but it was not granted.
That was "very frustrating," Glenn said, but he was hopeful publication of the study in the NEJM "will help encourage regulators here and around the world to find a way to get lambda into patients as soon as possible."
S.Abdullah--SF-PST




