
-
 Swiatek beats Paolini to clinch WTA Cincinnati Open title
Swiatek beats Paolini to clinch WTA Cincinnati Open title
-
Brazil's top court rules US laws do not apply to its territory

-
 Suits you: 'Fabulous' Zelensky outfit wows Trump
Suits you: 'Fabulous' Zelensky outfit wows Trump
-
Pro-Trump outlet to pay $67 mn in voting defamation case

-
 Downton Abbey fans pay homage to 'beautiful' props before finale
Downton Abbey fans pay homage to 'beautiful' props before finale
-
Republican-led states sending hundreds of troops to US capital

-
 Putin and Zelensky set for peace summit after Trump talks
Putin and Zelensky set for peace summit after Trump talks
-
UN debates future withdrawal of Lebanon peacekeeping force

-
 Trump says arranging Putin-Zelensky peace summit
Trump says arranging Putin-Zelensky peace summit
-
Hurricane Erin douses Caribbean, menaces US coast

-
 Sinner vows to play US Open after Cincy retirement
Sinner vows to play US Open after Cincy retirement
-
'Ketamine Queen' dealer to plead guilty over Matthew Perry death

-
 Leeds beat Everton for perfect start to Premier League return
Leeds beat Everton for perfect start to Premier League return
-
'Ketamine Queen' to plead guilty over drugs that killed Matthew Perry

-
 Guirassy sends struggling Dortmund past Essen in German Cup
Guirassy sends struggling Dortmund past Essen in German Cup
-
Stocks under pressure as Zelensky-Trump talks underway

-
 Alcaraz wins Cincinnati Open as Sinner retires
Alcaraz wins Cincinnati Open as Sinner retires
-
Trump floats Ukraine security pledges in talks with Zelensky and Europeans

-
 Doak joins Bournemouth as Liverpool exodus grows
Doak joins Bournemouth as Liverpool exodus grows
-
Excessive force used against LA protesters: rights group

-
 Panama hopes to secure return of US banana giant Chiquita
Panama hopes to secure return of US banana giant Chiquita
-
'Things will improve': Bolivians look forward to right's return

-
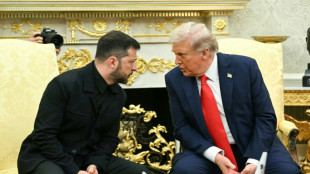 Trump welcomes Zelensky with fresh optimism on peace deal
Trump welcomes Zelensky with fresh optimism on peace deal
-
Israeli controls choke Gaza relief at Egypt border, say aid workers

-
 Air Canada flight attendants vow to defy latest back-to-work order
Air Canada flight attendants vow to defy latest back-to-work order
-
Hurricane Erin drenches Caribbean islands, threatens US coast

-
 Europeans arrive for high-stakes Trump and Zelensky talks
Europeans arrive for high-stakes Trump and Zelensky talks
-
Trump, Zelensky and Europeans meet in bid to resolve split over Russia

-
 Hamas accepts new Gaza truce plan: Hamas official
Hamas accepts new Gaza truce plan: Hamas official
-
Stocks under pressure ahead of Zelensky-Trump talks

-
 Russian attacks kill 14 in Ukraine ahead of Trump-Zelensky talks
Russian attacks kill 14 in Ukraine ahead of Trump-Zelensky talks
-
Lassana Diarra seeks 65 mn euros from FIFA and Belgian FA in transfer case

-
 Air Canada flight attendants face new pressure to end strike
Air Canada flight attendants face new pressure to end strike
-
Alonso says 'no excuses' as Real Madrid prepare for La Liga opener

-
 Deadly wildfires rage across Spain as record area of land burnt
Deadly wildfires rage across Spain as record area of land burnt
-
Swedish ex-govt adviser goes on trial over mislaid documents

-
 Injured Springboks captain Kolisi out for four weeks
Injured Springboks captain Kolisi out for four weeks
-
Irish literary star Sally Rooney pledges UK TV fees to banned pro-Palestine group

-
 Stocks mixed ahead of Trump-Zelensky talks
Stocks mixed ahead of Trump-Zelensky talks
-
Son of Norway princess charged with four rapes

-
 Fresh Pakistan monsoon rains kill 20, halt rescue efforts
Fresh Pakistan monsoon rains kill 20, halt rescue efforts
-
Forest sign French forward Kalimuendo

-
 Zelensky warns against 'rewarding' Russia after Trump urges concessions
Zelensky warns against 'rewarding' Russia after Trump urges concessions
-
FIFA boss condemns racial abuse in German Cup games

-
 Stocks diverge ahead of Trump-Zelensky talks
Stocks diverge ahead of Trump-Zelensky talks
-
Spain and Portugal battle wildfires as death toll mounts

-
 Joao Felix says late Jota 'will forever be part of football history'
Joao Felix says late Jota 'will forever be part of football history'
-
Javelin star Kitaguchi finds new home in small Czech town

-
 Rain halts rescue operation after Pakistan floods kill hundreds
Rain halts rescue operation after Pakistan floods kill hundreds
-
Zelensky says Russia must end war, after Trump pressures Ukraine


Moderna to Showcase Extensive Infectious Disease Research at ESCMID 2025
CAMBRIDGE, MA / ACCESS Newswire / April 7, 2025 / Moderna, Inc. (NASDAQ:MRNA) today announced that the Company will present research across multiple infectious disease areas, including COVID-19, influenza, respiratory syncytial virus (RSV), cytomegalovirus (CMV), norovirus, and mpox, at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Global Congress in Vienna, Austria, from April 11-15, 2025.
Moderna will present twelve scientific presentations at the ESCMID 2025 Global Congress, including three oral presentations, one e-poster presentation, and eight poster presentations, highlighting the breadth of its research in respiratory and emerging infectious diseases.
COVID-19
Immunogenicity of JN.1- and KP.2-encoding mRNA COVID-19 vaccines against JN.1 SARS-CoV-2 sublineages in adult participants - Oral Presentation
Presenter: Frances Priddy
Session: Advances in vaccine effectiveness for viral respiratory infections
Date: April 12 | Time & Location: 11:00 (Hall 9)Relative vaccine efficacy of a SARS-CoV-2 spike receptor-binding and N-terminal domain COVID-19 vaccine versus mRNA-1273: subgroup analyses - Oral Presentation
Presenter: Spyros Chalkias
Session: Vaccines and prophylaxis against respiratory viral infections
Date: April 13 | Time & Location: 11:00 (Hall 9)Clinical evaluation of a SARS-CoV-2 spike receptor-binding and N-terminal domain COVID-19 vaccine - Poster Presentation
Presenter: Spyros Chalkias
Session: COVID-19
Date: April 12 | Time & Location: 12:00 (Poster Hall)Immunogenicity and safety of a SARS-CoV-2 N-terminal domain and receptor-binding domain monovalent XBB.1.5 vaccine in Japanese participants aged ≥12 years - Poster Presentation
Presenter: Christina Grassi
Session: COVID-19
Date: April 12 | Time & Location: 12:00 (Poster Hall)
RSV
Six-month Immunogenicity of mRNA-1345 RSV Vaccine in Adults Aged ≥60 Years - E-Poster Presentation
Presenter: Mihir Desai
Session: On the frontiers of vaccine-driven prevention
Date: April 12 | Time & Location: 08:30 (Arena 1)Safety and immunogenicity of mRNA-1345 revaccination at 24 months in adults ≥60 years - Poster Presentation
Presenter: Mihir Desai
Session: Clinical Trials
Date: April 15 | Time & Location: 12:00 (Poster Hall)Burden of chronic medical conditions that are risk factors for severe RSV among adults aged 18-59 years in the United States - Poster Presentation
Presenter: Clarisse Demont
Session: Influenza and respiratory viruses
Date: April 12 | Time & Location: 12:00 (Poster Hall)Association of virology swab positivity in adults aged ≥40 years with Computerized Medical Record Reported Acute Respiratory Infection (ARI) Subgroups: Observational Study of ARI (ObservatARI) - Poster Presentation
Presenter: Simon De Lusignan
Session: Influenza and respiratory viruses
Date: April 12 | Time & Location: 12:00 (Poster Hall)
Mpox
Safety and immunogenicity of mRNA mpox vaccine candidate mRNA-1769: Interim analysis results from a Phase 1/2 Trial - Oral Presentation
Presenter: Hiwot Hiruy
Session: Novel vaccines in clinical development
Date: April 14 | Time & Location: 17:30 (Hall 3)
Cytomegalovirus
Long-term safety and immunogenicity of cytomegalovirus mRNA-1647 vaccine in healthy adults: 36-month results from a phase 2 extension trial - Poster Presentation
Presenter: Ben Lorenz
Session: Clinical Trials
Date: April 15 | Time & Location: 12:00 (Poster Hall)
Influenza
Six-month persistence and safety of mRNA-based influenza or multicomponent influenza/COVID-19 vaccines versus licensed comparators in adults aged ≥18 years - Poster Presentation
Presenter: Mieke Soens
Session: Influenza and respiratory viruses
Date: April 12 | Time & Location: 12:00 (Poster Hall)
Norovirus
Incidence and severity of sporadic, medically attended norovirus infection in the United States, 2023-2024 - Poster Presentation
Presenter: Mark A. Schmidt
Session: Viral epidemiology - general, prevalence studies, molecular and genomic epidemiology
Date: April 12 | Time & Location: 12:00 (Poster Hall)
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the potential for Moderna's vaccine candidates to alleviate disease outcomes. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and in subsequent filings made by Moderna with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Luke Mircea-Willats
Senior Director, International Communications
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna Inc.
View the original press release on ACCESS Newswire
Y.Shaath--SF-PST

